

Medical device manufacturers deal with various challenges to manufacture and market medical devices. These challenges range from competitive markets, stringent adherence to regulations, and maintaining high-quality product development. Among these challenges, developing an effective risk management strategy is paramount. One of the subsects of developing an effective risk management strategy is Failure Mode and Effects Analysis, or FMEA.
FMEA was first conceptualized in the 1940’s by the US Military but since then, it has been adopted, to implement safety and efficacy in various industries. In the medical device industry, it is implemented to identify and eliminate possible risk scenarios for devices ranging from diagnostic tools to implantable devices.
FMEA enables medical device manufacturers to anticipate failure by identifying all of the possible failure modes in a design or manufacturing process. If conducted correctly, it’s an excellent foundation tool for risk management. In this article, we discuss the role of FMEA in medical device development and risk management strategy.
Failure modes are the several ways in which a process can fail and effects are the various ways, the failure modes can impact the outcomes. FMEA is designed to identify, prioritize, and restrict failure modes.
FMEA, abbreviated for Failure Mode and Effects Analysis is an organized and structured approach to discover potential failures associated with a device, component, or a part. The primary goal of failure mode and effects analysis is to identify and assess potential failure modes, estimate their severity, and mitigate the risks associated with the possible failure.
FMEA enhances manufacturing technologies by applying the expertise and knowledge of a cross-functional team to review the design by assessing its risk of failure.
An extremely structured approach needs to be followed to conduct FMEA. Performing FMEA requires excellent collaboration within the FMEA team. Depending upon the medical device’s complexity, various brainstorming sessions would be required to conduct and complete the FMEA process.
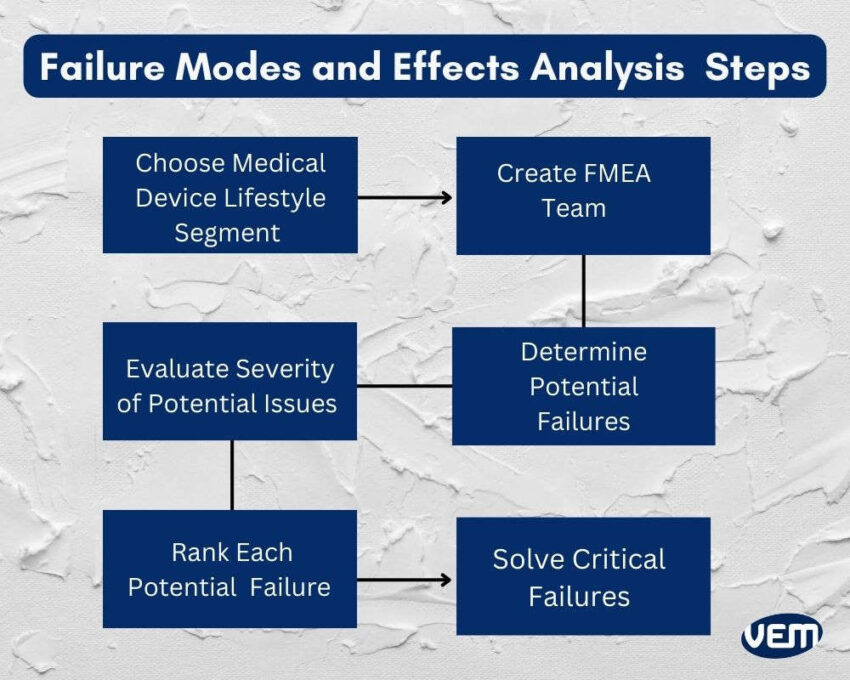
The first step is to select the specific aspect of the medical device lifecycle that requires analysis such as the device design, manufacturing, clinical use, etc.
The next step is to create an FMEA team comprising professionals with expertise in biomedical engineering, regulatory affairs, quality management, and risk assessment. The FMEA team must include subject matter experts who are thorough with the device knowledge and its use.
The specific aspect of the medical device lifecycle is thoroughly reviewed in the subsequent step, which helps identify and evaluate potential failure modes. These failure modes can affect the integrity of the device and the patient’s safety.
After the aspect is thoroughly reviewed, the severity of each potential failure is determined for patient safety, functionality, and regulatory compliance.
The Risk Priority Number (RPN) is noted for each failure mode. It is calculated by multiplying its severity, occurrence, and detection ratings. The RPN enables one to understand the perceived risk level and prioritize risk mitigation activities accordingly.
Let’s understand the various types of FMEA that are employed for medical device risk analysis:
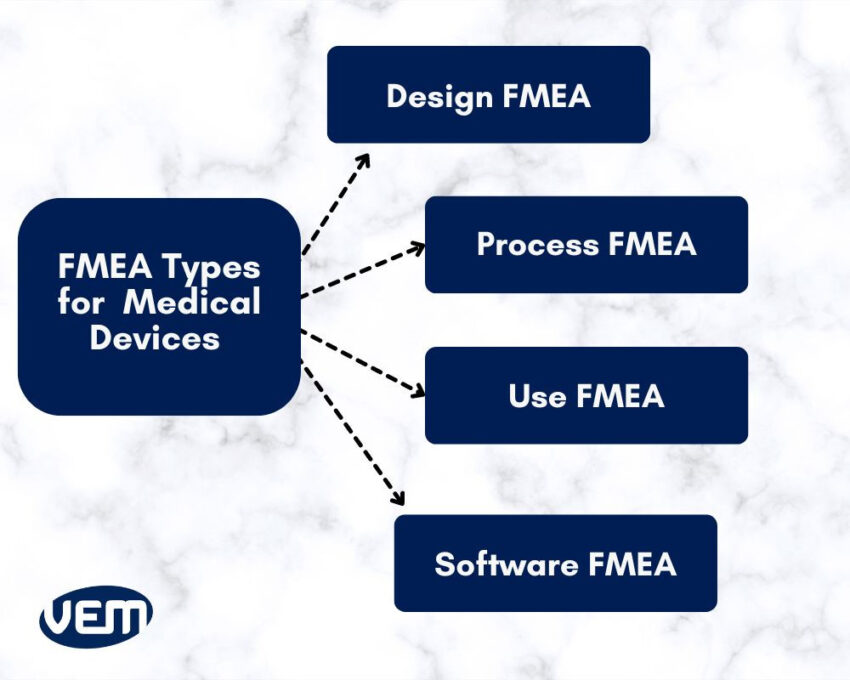
DFMEA, abbreviated for Design FMEA identifies and mitigates various failure modes during the design and development phase. It aims to improve upon the design process and ensure that all parameters of the regulatory bodies are met.
This method analyzes all failure modes that are possible for each design component. It also aims to identify how the component can fail to perform its function and thereby affect the end user.
PFMEA, abbreviated for Process FMEA identifies various potential failure modes within the equipment and manufacturing processes. It helps manufacturers analyze how the process settings introduce risks and affect the end user.
The primary goal of process FMEA is to avoid releasing a non-compliant or defective device.
UFMEA, abbreviated for Use FMEA identifies failure modes when the medical device is in use in healthcare settings. This type of failure mode occurs when the design fails to perform as intended. It primarily evaluates the operation of the devices during clinical use.
Software FMEA identifies and assesses failure modes within the software components that are integrated into medical devices. It primarily evaluates software technology that is embedded in either diagnostic equipment or therapeutic devices.
FMEA can be performed occasionally or continually throughout the lifetime of a medical device. It can be performed in the following case scenarios:
Reliability, accuracy, and patient safety are crucial aspects of the medical device industry thus, FMEA plays a significant role! Let’s understand the significance of FMEA in manufacturing medical devices:
It helps manufacturers identify various potential failure modes and their effects, enabling them to develop risk mitigation strategies. You should note that the risk management file is a crucial component to obtaining market access in the industry.
It’s also an effective tool for identifying process failures early on. It, therefore, corrects consequences due to poor performance. It also helps to verify and validate changes through early discovery of failure.
FMEA increases medical devices’ accuracy, sensitivity, and reliability through its thorough analysis. It proactively identifies the possibility of malfunction issues in medical devices during the design and development stages thereby, increasing patient care.
It improves design for manufacturing and assembly (DFM/A) and enables collaboration between design and operations. In addition, FMEA continually improves manufacturing and post-marketing processes directly or indirectly.
Let’s understand some of the most common mistakes that are made while implementing an FMEA:
FMEA should be started as early as possible. It has the most impact when implemented early in the design process.
FMEAs mustn’t be used as an emergency fix and applied in time to ensure an apt impact on the operations. It is a risk management tool that can be used to detect failure modes as early as possible in the design and development process.
The focus should always be on a specific aspect of the medical device lifecycle. If the scope is too large, then it can be overwhelming and one can draw incorrect conclusions. In addition, the process shouldn’t be overcomplicated. You should note that streamlining the process avoids problems later on.
FMEA results must be correctly analyzed to ensure that the medical device design and development process is on the right track. Thus, it is imperative to objectively analyze your results and incorporate clear communication to avoid ambiguity.
Let’s understand some of the risks that can arise from various sources:
There are three risk concepts to calculate potential risks associated with failure mode effect analysis. The risk concepts are scored on a scale of 1-10 i.e. from lowest to highest. The number obtained for each risk concept is multiplied to achieve the risk priority number (RPN). RPN is directly proportional to the risk priority i.e., the higher the risk priority, the higher the risk priority number. Let’s understand these concepts further:
The severity is a failure mode’s consequence. Failures such as cosmetic defects are typically considered low severity whereas failures that affect functionality are considered high severity.
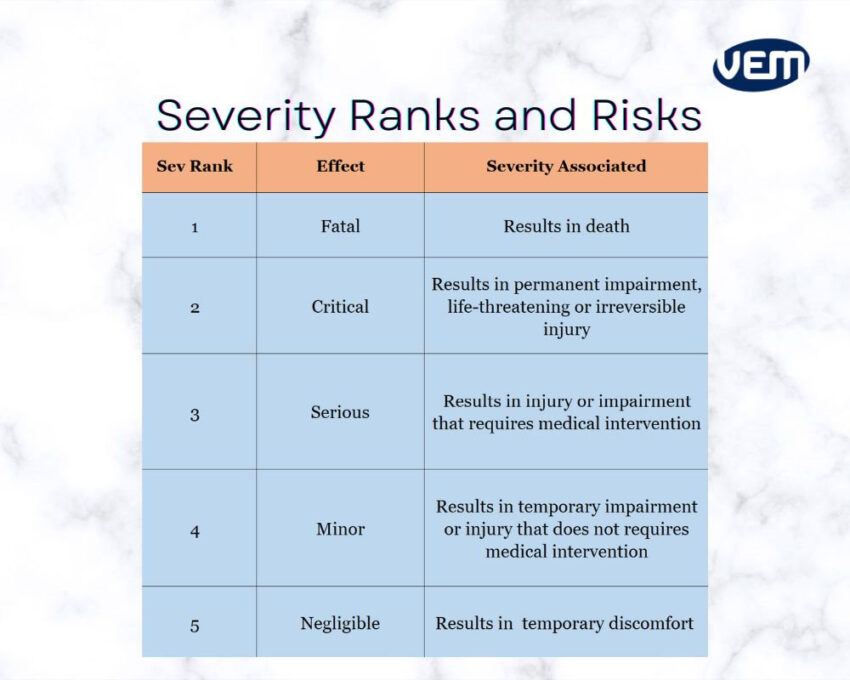
Occurrence refers to the probability of a failure mode occurring. If the occurrence is unlikely, then it is considered to have a low occurrence score whereas if the occurrence is likely to occur, then the result would be a higher occurrence score.
The chance of detection is the probability of detecting failure modes. This score detects if the current processes can detect failure. If the failure cannot be detected from your current processes, then the score is higher.
A risk evaluation matrix includes severity and probability of occurrence levels. The color coding denotes the various risk levels. An example of a risk evaluation matrix is below:
This is an electrochemical process that is typically employed to round the edges. It also helps to remove damage from slag, burrs, and heat-affected material.
In this process, the nitinol components are connected to an electric current and they are placed in an acid solution for a predetermined time frame.
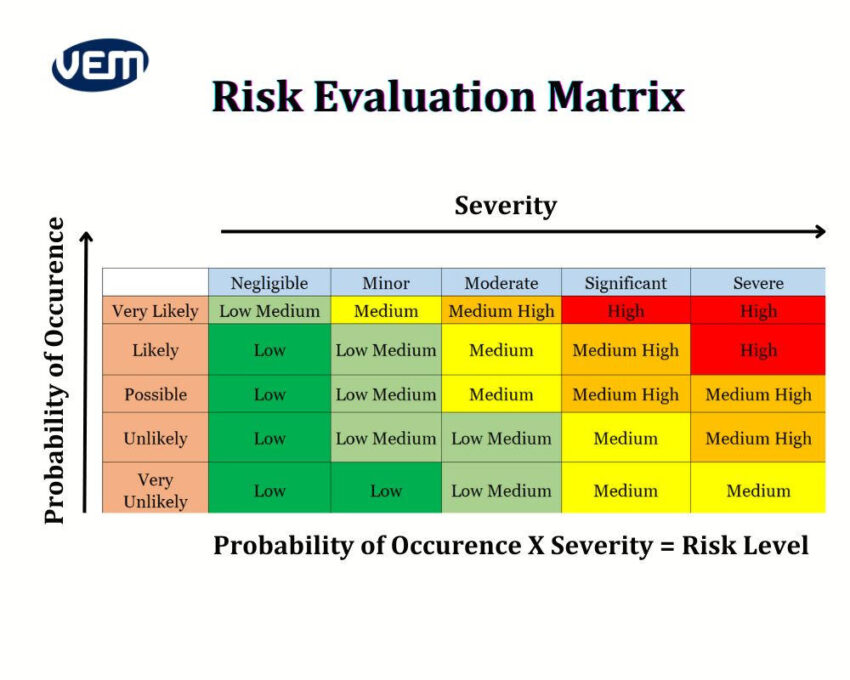
Risk levels that are high or unacceptable require a risk reduction and a possible risk/benefit analysis.
Quality assurance is an integral part of manufacturing medical devices and it is thus, critical to ensure that the right process is implemented to carry out the risk analysis and management.
Risk management is required by most regulatory authorities for manufacturing and marketing medical devices. You should note that it is one of the most critical aspects of quality assurance and regulatory compliance for medical devices.
Failure mode and effects analysis is a great tool, but it also has limitations and doesn’t cover every aspect of risk management. FMEA is only a subsect of the strategy and doesn’t include risk management planning.
The strategy of risk management implements policies and procedures to identify, evaluate, and control risks. It includes tackling all types of hazards and risks. FMEA on the other hand, is much narrower in its scope and specifically focuses on identifying and evaluating possible failure modes. FMEA is thus an integral and necessary aspect of risk management but also, requires other processes to be implemented.
Medical device manufacturers are required to have a robust risk analysis and management system. The key regulations and standards are listed below:
To reduce risks, it is essential to identify and evaluate the possibility of risks. To estimate the same, the following tools are recommended:
As you can see from the above structure, FMEA is focused on failure mode prioritization but it doesn’t focus on elimination of those risks. Thus, FMEA is a part of a larger risk management system.
FMEA serves as a valuable tool for medical device risk analysis by empowering medical device manufacturers to identify, evaluate, and mitigate potential risks. To ensure regulatory compliance and patient safety, it is crucial to adhere to a structured process.
VEM Medical has over 20 years of experience in manufacturing medical devices. We can help you with developing risk management strategies including FMEA methodologies and more.