

Plastic parts such as medical devices and equipment need to be manufactured in cleanrooms as they need to be sterile. That means protection from dust, chemical vapors, aerosol particles, airborne microbes, and other contaminants. It is important that product quality, integrity, and safety are not compromised under any circumstance which is why it is essential to maintain cleanrooms. In this article, we explain in detail how to maintain cleanrooms for manufacturing.

In order to maintain cleanroom standards, it is essential to follow cleanroom protocols! Responsible injection molders that provide services to medical device manufacturers ensure that they follow and adhere to the stringent protocols of cleanroom standards. Injection molders must ensure that the various variables are maintained in order to meet compliance.

It is imperative that Clean rooms control various types of environmental conditions such as humidity, temperature, and pressure to ventilation. Maintaining a cleanroom for injection molding and manufacturing medical devices is optimized by ensuring the following:
We have discussed these protocols and various environmental personnel guidelines in detail in this section:
The first parameter that must be paid attention to is the quality control systems, such as HEPA filters and machine hoods. These quality control systems monitor, manage and remove particulates and dust particles. If this is not controlled, it can cause pollution particles to enter the environment which could result in the fallout of the specifications set for ISO 7 and ISO 8 clean room standards.

People can naturally introduce contaminants such as skin sheds, oils, sweat, and hair into the cleanroom environment thus, it is imperative that precautions are taken to avoid these contaminants from entering the cleanroom. In addition, human movement and behavior also need to be controlled in order to maintain the cleanliness of the cleanroom. We have listed some personnel guidelines that are necessary to keep a controlled environment so that the cleanroom environment is not put at risk:

Most cleanroom environments require a relative humidity level to be maintained between 30% – 40% RH with an air temperature of 21°C / 69.8°F. This is generally an ideal environment to avoid many negative repercussions such as bacterial/fungal growth, corrosion, and static electricity. It is also ideal for employee comfort because if they tend to sweat or shiver, they may release more particles into the environment.
It is imperative to regulate the airflow in order to maintain a clean room. There are 2 types of air flows that are regulated to avoid the risk of contamination.
Positive pressure air flow is created when clean, filtered air is pumped through the ceiling into the cleanroom and is generally used to keep contaminants out of the cleanroom. If there is a leak, positive pressure airflow forces clean air into the cleanroom.
On the other hand, in a negative pressure cleanroom, all the outlets i.e. the windows and the doors are completely sealed. Thus, a lower pressure is created within the room. This results in outside air flowing into the cleanroom. Negative pressure is applied when you want to keep any possible contamination escaping from the cleanroom.
One of the most overlooked sources of contamination is common office supplies, such as mouse pads, notebooks, sticky notes, paper, and ID badge holders. Moving a pen from an office environment to a cleanroom can cause contamination thus, it’s important that these materials remain in your cleanroom. It’s also best not to post anything on the cleanroom walls, such as sticky notes as it can shed particles.
The supply packaging that is used may not always be compliant. For instance: If you order nitrile cleaning gloves that are cleanroom-compliant, the cardboard box that dispenses them probably isn’t, as it could release particulate into the air thus, these errors need to be paid attention to.
It is important to ensure that the right cleaning materials are used to avoid the risk of contamination. Simply using a paper towel or cloth can compromise a cleanroom thus, wipes, swabs, and other cleaning supplies should be carefully evaluated for use in cleanrooms and as per the specific ISO Class. It’s also necessary to keep exclusive cleaning equipment such as brooms and mops. These supplies should be exclusive to your cleanroom and not used elsewhere in the facility.
While procedures often cover standards for personal hygiene, it’s important that the cleanroom workers avoid make-up, perfume, and jewelry which are potential contaminants. Items such as food, beverages, candy, or gum should not be brought into the cleanroom.
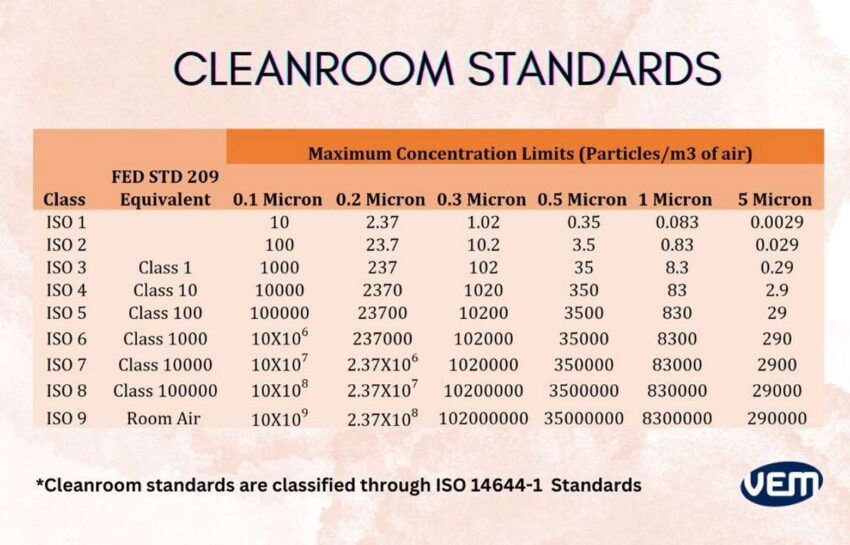
Cleanroom standards are classified through ISO 14644-1 and it is defined by the degree of purity of the air which is based upon particle concentration per m³. This system classifies the cleanrooms through ISO 1-9 in which the highest purity is that of ISO class 1, whereas the lowest is ISO class 9. The following table depicts the Cleanroom Standards:
If you would like to learn more about cleanrooms, you can view this article where we explain in detail the requirements of cleanrooms.
Our plastic injection molding and tooling facility in Thailand has various cleanrooms. Our team in Thailand has the expertise and is dedicated to providing you with a great manufacturing experience and exemplary service. Contact us and a team member will connect with you to resolve your queries.