

The design and development of medical devices is a complex and multi-step process. It has various sequential stages which are listed below:
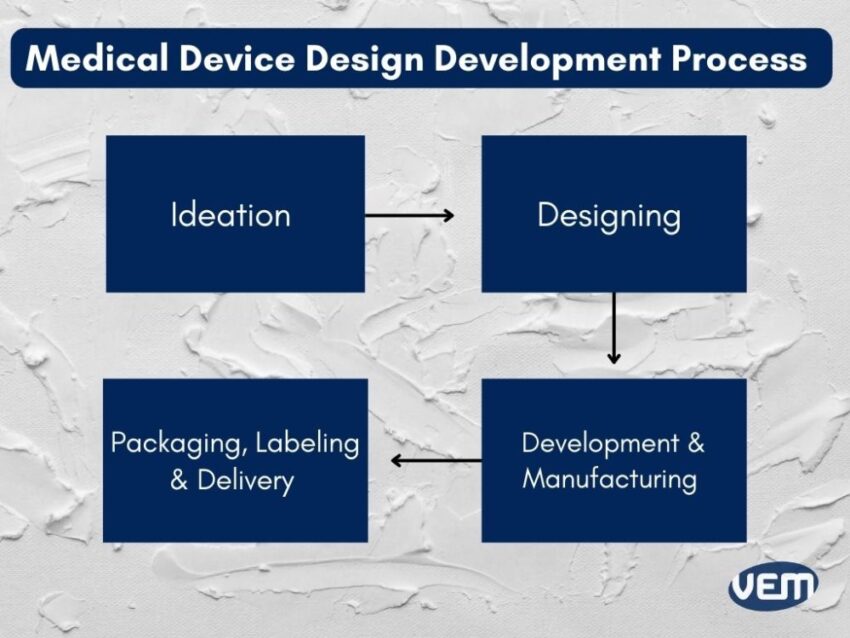
The development of medical device design is the most preliminary step and is crucial for creating a medical device that is specific for the intended use. The medical device design that you aim to develop should be such that it’s adaptable to sustain in the competitive market. It should add value to the end user and capture a profitable section of the market share.
The medical and healthcare industry deals with life-critical segments that often involve complex procedures. Thus, the development of any medical device should not only be solution specific but should also be aligned with healthcare regulatory requirements. It should seek a holistic approach and include the complete process. The design and development process of medical devices requires precision in every stage! This article is a comprehensive and definitive guide to successfully designing and developing medical devices that address customer requirements.
The medical and healthcare industry deals with life-critical segments that often involve complex procedures. Thus, the development of any medical device should not only be solution specific but should also be aligned with healthcare regulatory requirements. It should seek a holistic approach and include the complete process. The design and development process of medical devices requires precision in every stage! This article is a comprehensive and definitive guide to successfully designing and developing medical devices that address customer requirements.
In general, the following aspects need to be kept in mind while designing and developing a novice medical device:
Missing out on any of these focus areas can lead to delays, significant financial loss, and product recalls thus, it’s critical that these areas are well understood.
Developing a progressive medical device design requires an elaborate scope definition, collaborative efforts across the team, and adherence to various specifications and requirements to ensure state-of-the-art quality. In addition, the manufacturer needs to ensure safety and mitigate risks.
Innovations in any industry commence with analyzing and identifying the market! These innovations could lead to design solutions that address an untapped, unmet requirement or they may simply address a much more efficient way of an already existing solution.
These solutions could range from a better way of monitoring health to providing enhanced care or they could be aimed at delivering better administration to the healthcare industry. It’s crucial to conduct a thorough evaluation of the requirements that need to be addressed in order to develop a solution. The following steps indicate how to start with product ideation:
The primary step is identifying the requirement and this depends on 2 factors:
The classification of medical devices and their pre-market approval is based on the risk that is associated with their use. You can read more about it here.
In addition, you also need to address compliance procedures and intellectual property rights. If a similar idea is formed, then it might actually disallow you to move ahead further with the development.
Once the idea has been developed, it is moved into the discovery phase. This phase consists of initial designing, prototyping, and iteration-driven redesign.
A strong team that is expert in engineering, design, quality assurance, and regulatory affairs is crucial to complete the discovery phase successfully. Once this phase has successfully been completed, you can proceed further for approval from the regulatory bodies.
The process of design control is holistic! It doesn’t simply start with designing in the initial stages nor does it end, as the design is transferred to the production phase.
If there are changes in the design phases, it impacts the various manufacturing processes! This is applicable even with post-production feedback.
Design control is an ongoing process as the goal here is to develop a product that is user usable! Thus, the product needs to undergo constant changes to implement feedback and update usage patterns. The following image depicts the design control process through the waterfall process:
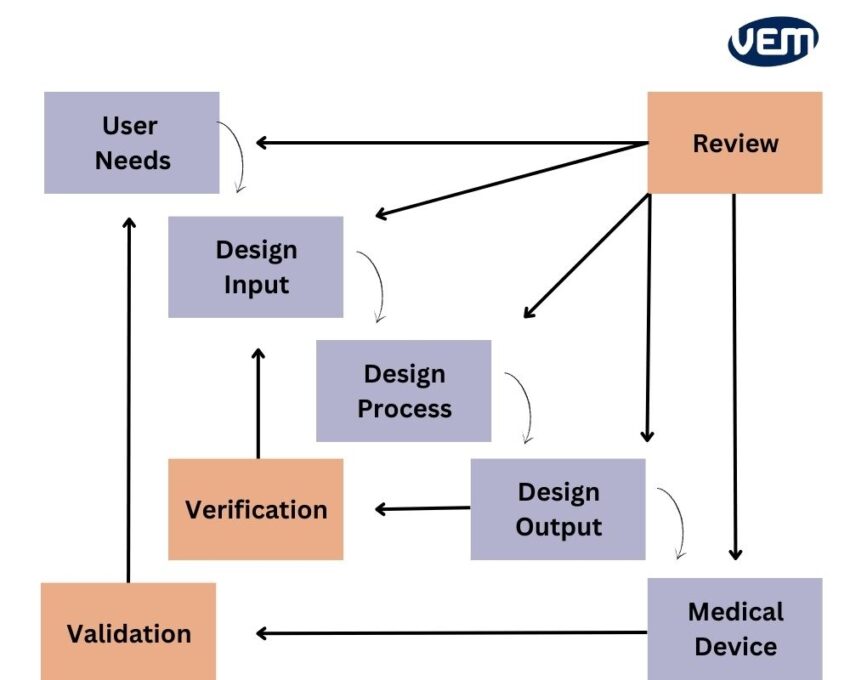
Design control ensures that the medical device complies with the requirements of the end user. This process entails analyzing various factors.
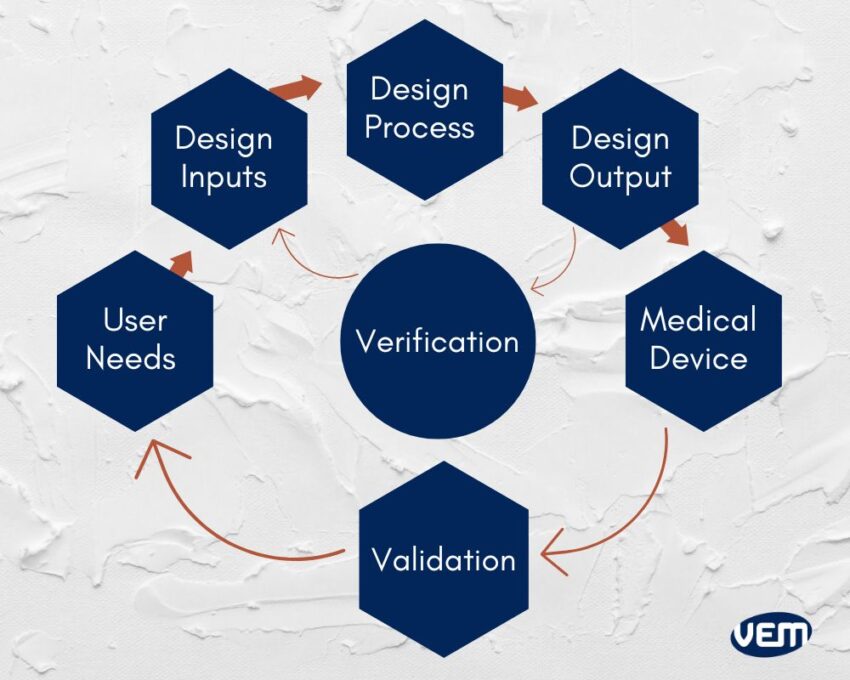
Let’s take an in-depth look at these factors:
User Needs is the starting point for the development of any medical device design. User needs need to be established extensively and all data needs to be documented. The market feedback is analyzed to understand and meet the market requirement. You should note that user needs is not just a preliminary step. The feedback should be taken during each and every step and modifications if required should be implemented in the medical device to meet the user’s needs. The best way to understand what the user needs are is by seeking answers to questions like:
Design input is an iterative process and is the commencement of converting user needs into device design. At this stage, the inputs of the requirements are reviewed and tested to understand if the design is acceptable.
At this stage, the design input is converted into high-level specifications which are then incorporated as features in Medical Devices. This is a transition process of converting the requirements from the design input to the output stage.
This stage involves transferring the data of the process into the production facility for prototyping. At this stage, it is mandated by the design control regulation to maintain DHF i.e. design history file. You should note that DHF must be accessible to all team members.
During the verification process, it is analyzed whether the specifications of the design meet the requirements or not. If the specifications need to be revised, the output becomes the input and this process continues until the output design is aligned with the input design.
Once the final design is ready, it is transmitted to the production facility for mass production. DHF at this stage, too illustrates all the linkages of the design controls and helps to trace changes throughout the entire product development process.
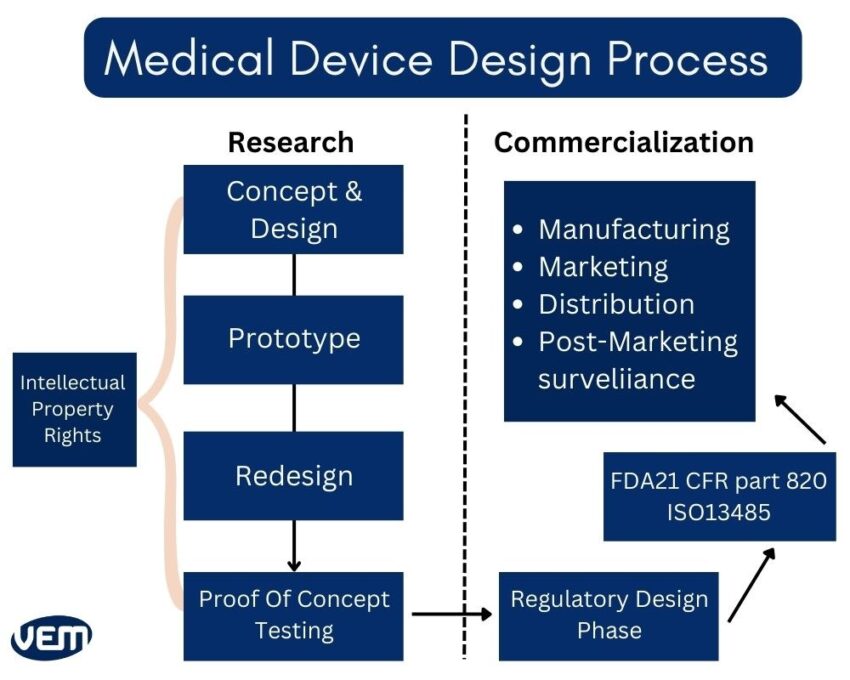
The first phase of medical device development is all about planning and researching! You should note it’s about developing products in a highly regulated medical device industry.
It is imperative that thorough market research is conducted. It enables you to discover if there are any similar devices in the market and understand if you need to conduct any types of clinical trials. If clinical trials need to be conducted, then the costs involved will be higher thus, it is crucial to conduct thorough research.
If your project is about developing a new product i.e. introducing an unprecedented concept, then you need to protect your intellectual property. You should consider patenting your idea.
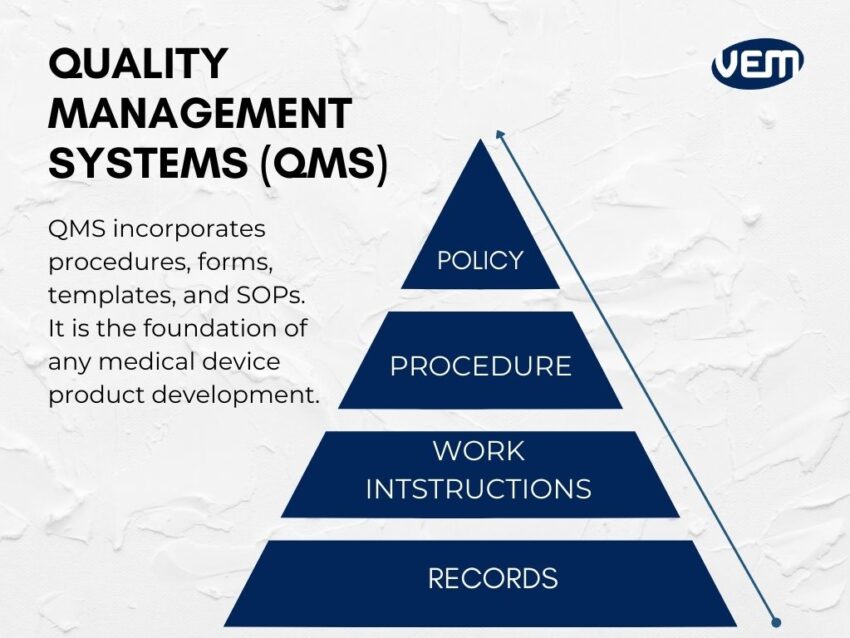
You should also think about implementing a quality management system (QMS). A QMS is the foundation of any medical device product development. It incorporates procedures, forms, templates, and SOPs. It not only provides a base to build on but also ensures that all compliances of the regulatory bodies are met. Your QMS basically controls all activities that are related to your medical device!
The main goal of this phase is about developing the concept and proving the concept. Phase 2 requires utmost delineation towards noting customer requirements and calculating risks.
In this phase, it is recommended to garner customer feedback via surveys and interviews. These insights, along with competition analysis and market research should be included in the product design. Once proof of concept has been established and your medical device has been positioned in the market, you can begin prototyping and if required, conduct clinical trials.
In addition, you should research the requirements for product submission. Although there is a major overlap in the regulatory processes of various regions, the product submission processes may be in a particular way for the country/region you plan to position your device in.
Phase 3 in medical device development focuses on the validation and verification processes. These processes indicate that your device will be able to withstand all the claims that are made. At this stage, you should make it a point to note the acceptance criteria for each test.
You can do this by setting up a design traceability matrix. This matrix depicts the various connections that exist between the requirements of the user & the specifications. It also elaborates how the user requirements should be validated and how the specifications should be verified. The design traceability matrix ensures that all the customer requirements are met.
It is also important to document all the potential failures in this phase. These could be due to poor design, incorrect manufacturing processes, or various types of user failures.
The next phase is about branding and marketing. You should note that if you are making a claim, you must back it up with substantial evidence. You should note that regulatory agencies have very strict requirements and consider marketing materials in the medical device industry to be a form of labeling. Quality System Regulation (QSR), 21 CFR Part 820 incorporates specifics for medical device quality management systems.
In this phase, all the data for validation and verification tests should be gathered. You should also document your testing evidence of biocompatibility, electrical safety, and stability. In addition, you should ensure that your technical documentation is complete for the regulatory agencies to review.
If your technical file review is complete and you are done with your product submission, then your medical device will be granted licenses/certifications and now, you can launch your product in the market.
You should make sure that you have a robust internal quality auditing system to review your records and ensure that you are following your QMS procedures and adhering to good manufacturing practices (GMP).
At this stage, you should be ready for post-marketing surveillance and be ready for customer feedback. Their suggestions can be implemented for implementing improvements.
The medical device industry has advanced significantly in the past 2 decades. Today, medical devices are developed with emerging technology advances to be smaller, and more accurate, and they are developed to gather and deliver data instantly such as wearable devices.
The medical industry is always seeking to support patients with maximum comfort and in a non-invasive manner! These goals are shaping into reality through engineering technologies that cannot only make the devices small and lightweight, but also smart, safe, and reliable.
In order to support these new systems, medical device manufacturers need to keep in mind various factors such as flexibility, light-weightlessness, and the capability of transmitting real-time patient data, and all of this should be powered within the allowed printed circuit board capacity to ensure that the devices are not necessarily bulky. You should note that these new technologies are often game-changers and set an unprecedented approach to developing healthcare systems. In order to achieve this, there are three main considerations that should be kept in mind when designing medical devices. Let’s take a closer look at some of the design considerations that should be integrated to ensure that the device you develop is advanced for today’s age.
Today’s technological advancements make it possible that medical devices are not only smart and sophisticated, but they are also compact. Your medical device designing team should be willing and eligible to accommodate this demand for smaller devices. One of the ways to do so is by optimizing board space with newer micro-connectors. An example of medical devices that need to be more compact than ever is wearable devices.

In addition to being smaller, it is important that new-age medical devices are also feature-rich. These features should capture more results that are accurate and various parameters as well. To incorporate more features, technologies such as flexible printed circuits (FPCs) should be adopted. FPCs are smaller, lighter, and more flexible!
One of the ways to manufacture a new-age medical device is by including board connections that have higher circuit counts of 60, 80, and 100 circuits. To attain maximum power in small devices, low-profile wire-to-board, and flex-to-board options are considered. These solutions can enable the functionality of a device by carrying up to 15 amps of current. Through these circuits, data is transmitted between connection points in a small form factor thus, these are becoming immensely popular!
The purpose of any medical device is to improve the quality of life. In order to keep patient and user risk to a minimum, there are various compliances by regulatory bodies that need to be adhered to! Our end-to-end solutions help you accelerate product development through collaboration and traceability. We also help you gain visibility throughout your product’s lifecycle.
At VEM-medical, we have experts in medical device manufacturing and engineering to help you develop and design state-of-the-art medical devices while ensuring compliance! If you would like to learn more about medical device design development, contact us and we will get back to you right away.