

Medical device sterilization is a key requirement of manufacturing medical device equipment. It is a standard procedure that ensures that medical devices are free from all types of life forms or organisms i.e. microbes, viruses, spores, fungi, etc.
Sterilization stems from the term sterile, which means that there is a complete absence of any viable organism. The sterilization procedure you choose for your medical device is dependent upon various factors.
While most medical devices are made of heat-stable materials and can thus, be sterilized via steam sterilization, many devices are also moisture-sensitive or may require low-temperature sterilization systems. In this article, we discuss various sterilization procedures that you can implement for your medical devices.
Sterility means that the medical devices are free from viable organisms. You should note that sterility cannot be proven and products are labeled sterile only if the contamination possibility after sterilization is less than 1 in 1 million i.e. SAL 10-6 or less.
Sterilization destroys all organisms in a medical device. The concept of what constitutes a particular part to be sterile is the probability of sterility after the device has been sterilized which is referred to as the sterility assurance level (SAL). Let’s understand the concept of sterility further.
SAL is defined as the probability of a single viable organism in a part after sterilization and is expressed as 10−n. For instance: If the probability of an organism surviving is 1 in 1 million, then the SAL would be 10−6.
Sterilization, Decontamination, Disinfection, and Cleaning are distinct aspects that have unique protocols. Let’s understand the role of each technique:
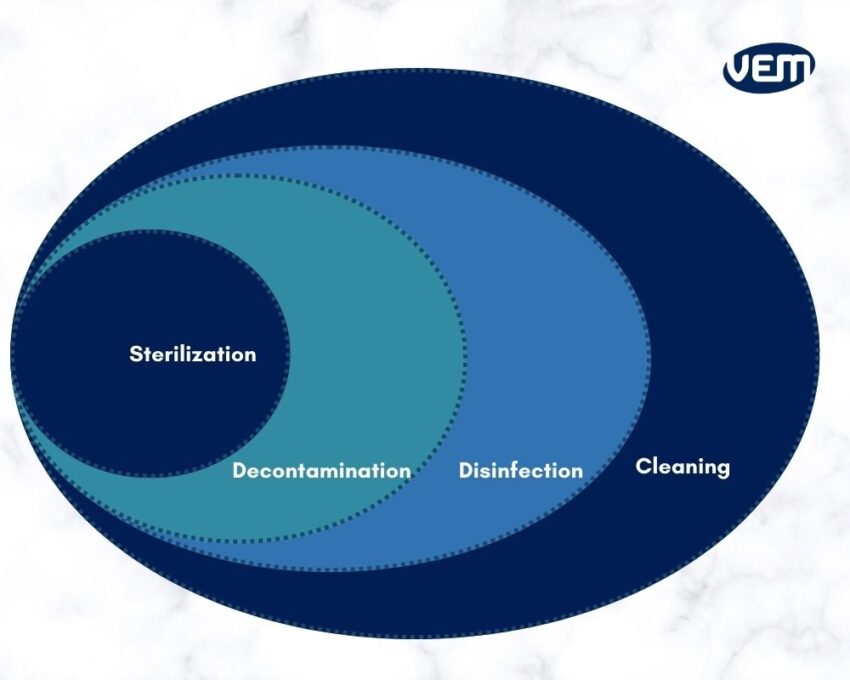
Sterilization is the process of applying a technique to the finished medical device to achieve a predetermined sterility assurance level (SAL) of 10⁻⁶.
Decontamination is eliminating all types of viable contamination to ensure that the environment is free of any bioburden.
Disinfection is the process of eliminating all types of organisms (except for bacterial spores) on surfaces with the help of liquid chemicals or wet pasteurization. The goal is to reduce the surface bioburden to a safe level.
The cleaning process is the removal of contamination and foreign material usually accomplished with the help of water and detergents. It is carried out to the extent that is necessary or for intended use.
There are various types of sterilization techniques for medical devices and each technique has unique properties. Let’s understand the various types of techniques:
Autoclave steam sterilization combines heat, humidity, and high pressure and is one of the most effective sterilization processes.
The autoclave is essentially a large pressure cooker. In this process, the parts are placed into the autoclave which is then sealed. Now, under very high pressure, high-temperature steam is pumped which displaces the air. The high steam kills the organisms and denatures enzymes as well as structural proteins. Once the sterilization time is complete, the steam releases and the parts are sterile. Typically, the recommended temperatures for autoclaving at 121° C / 250° F, 132°C / 270°F or 135° C / 275°F.
Autoclaving should be reserved for parts and products that can withstand humidity, high pressure, and high temperature. For instance: Surgical instruments can generally withstand temperatures of up to 125°C/257°F.
In dry heat sterilization, hot air is blown inside an industrial oven chamber to deactivate all life forms. Typically, the dry heat setting cycles are 160°C / 320°F for 2 hours, 170°C / 340°F for 1 hour, and up to 190°C / 375°F for 6 to 12 minutes. These cycle times vary and are dependent upon the type of application, oven type, and the blower unit. The heat denatures the proteins thereby eliminating all forms of biological agents. Dry heat sterilization penetrates well and is thus able to achieve an in-depth sterilization effect.

Dry heat ovens are relatively inexpensive and there are no toxic or environmentally hazardous agents involved in this sterilization process. When compared to other techniques, dry heat sterilization is more time-consuming than steam, chemical sterilization, or radiation. Overexposure to high temperatures of dry heat can irreversibly damage the structure of parts.
The ETO sterilization process uses a colorless gas, ethylene oxide (EO) to sterilize various medical devices. This process has three segments i.e. Preconditioning, Chamber & Aeration.
ETO is a very efficient process for the sterilization of medical devices. During the preconditioning stage, air is removed to allow the gas to enter the chamber, and within the chamber; the rotary pumps are designed such that they maintain an optimum ETO concentration. The chamber is heated by steam which ensures that the sterilizer is completely airless.
ETO sterilization process can be customized for various medical devices and is particularly apt for parts that cannot withstand the high temperatures and humidity of autoclave sterilization. ETO is also well-suited for medical devices with embedded electronics as the temperature conditions are maximum up to 60° C / 140°F. However; ETO is a highly combustible gas, and thus caution must be exercised during this sterilization process.
Chlorine dioxide, abbreviated as CD was discovered in 1811 and designated as a sterilant in 1988. The CD sterilizer process involves a container, where the medical devices that need to be sterilized are placed. The CD sterilization process has 5 stages which are listed below:
The sterilized parts can be removed from the container after the aeration is complete. CD gas sterilization takes approximately two to three hours to complete. Chlorine dioxide is an oxidizing chemical that causes cell disintegration by reacting with biological components such as cell membranes. CD interferes with the structure of organisms and disrupts their enzymatic functions. Its efficacy is primarily due to its oxidative attack on several proteins of the organism.
This sterilization process is ideal for parts that cannot withstand the high temperatures and humidity of autoclave sterilization.
Hydrogen peroxide creates reactive oxygen species such as hydroxyl radicals. These oxygen species create oxidative stress and attack to disintegrate nucleic acids, enzymes, and cell wall proteins.
Vaporized hydrogen peroxide sterilization process typically has three stages i.e. Conditioning, Hydrogen peroxide injection, and aeration. The entire sterilization process takes approximately 1-2 hours to complete and once, the aeration is complete, the medical devices are sterile and removed.
Just like CD and ETO Sterilization, VHP sterilization is also apt for medical devices and parts that cannot withstand the high temperatures and humidity of autoclave sterilization.
During the plasma phase of the cycle, hydrogen peroxide plasma sterilization combines hydrogen peroxide gas with free radicals such as hydroxyl and hydroperoxyl free radicals to denature the organism. Typically, the hydrogen peroxide plasma sterilization process takes between 1-3 hours to complete and consists of four steps:
The temperature process range is between 40° to 65°C / 104° to 140°F and while this is an acceptable range, the 13.56MHz RF energy of 200W to 400W interferes with the embedded systems during the plasma discharge phase. Thus, hydrogen peroxide plasma sterilization should not be used for medical devices containing semiconductors.
Gamma irradiation is a sterilization procedure that is popularly used to sterilize medical devices and it makes use of Cobalt-60 to denature the DNA thereby, killing the organisms.
In this sterilization process, medical devices are placed in their sealed packages and they are then placed in specific pallets which are now placed on a conveyor belt. The conveyor transfers devices in the radiation field and the parts are exposed to high-energy gamma rays that are produced via Cobalt-60 radioactive decay.
The radiation creates free radicals which are highly reactive oxidizing (OH, HO2) and reducing (H) chemicals. These chemicals disintegrate the vital components such as enzymes and DNA ultimately leading to cell death. To sterilize medical devices, sterilization levels are typically employed in the range of 250 to 500 rads. In this range, semiconductor devices can also operate and thus, gamma ray sterilization can be used to sterilize medical devices embedded with semiconductor electronics and sensors.
Gamma radiation is extremely reliable and accurate. Gamma rays have a very high penetration power, thus, medical devices can be sterilized in their final packaging.
Electron beam referred to as E-beam is another type of sterilization technique that can be used to sterilize medical devices with their final packaging.
Electron beam radiation does not penetrate as well as gamma rays but is faster than gamma-ray sterilization and occurs at a higher room temperature at normal atmospheric pressure.
E-beam sterilizes by accelerating an electron beam to near-light speed which further passes through a scanning chamber. The medical devices are placed on the conveyor and they move through this chamber and are exposed to high-energy electrons that further penetrate the device. Typically, energy levels of 5MeV to 10MeV are required to achieve the penetration required for sterilization. E-beam radiation produces free radicals that react with macromolecules and denature DNA.

The type of sterilization technique you choose for your medical device should be based on the intended use of the device and the type of material. The most popular sterilization process is steam sterilization and the second most popular technique is ethylene oxide gas sterilization which is often used for heat-sensitive materials.
If the medical device is embedded with electronics and without batteries, then the most effective and best sterilization technique is ethylene oxide and vaporized hydrogen peroxide. In addition, Chlorine dioxide has an electronic component compatibility as it does not pose any negative effects.
Radiation is a cold type of sterilization process that is very effective but it cannot be used on materials that might break down upon radiative exposure.
Choosing a sterilization technique is a complex process as the process you choose must be per the material type.
At VEM-Tooling, we can help you choose and offer solutions for some of the most common to complex medical device sterilization techniques. Our team is experienced in manufacturing and delivering sterile, high-quality medical devices. Contact us to learn about which medical device sterilization method is right for your product.