Today, medical plastic parts are revolutionizing the healthcare industry! They are continually evolving and becoming more and more advanced in the global market. This significant impact is due to the stride development of medical polymers.
Medical polymers have transformed medical devices by replacing various materials such as glass, metals, ceramics, etc. Medical plastics demonstrate distinct characteristics and enhanced capabilities. They have improved medical devices to be more durable, lightweight, bio-compatible, and cost-effective.
Today, medical polymers are applied to an array of applications in the medical device industry. They are employed to manufacture everything from medical tubes and syringes to complex implants and monitoring machines. In this article, we discuss in detail medical plastics, their various types, applications, and consideration factors.
What are Medical Plastics or Medical Polymers?
Medical plastics, also known as medical polymers undergo various screening procedures to ensure that they can be applied to manufacture a wide array of medical plastic parts.
There are various types of medical plastics and each varies distinctively in their properties and characteristics.
Medical plastics are subjected to exacting requirements by the regulatory bodies which is why they are employed for manufacturing medical plastic parts and medical devices.

Significance of Medical Plastics
Medical plastics are an excellent material of choice due to their distinctive properties. They are biocompatible, durable, and can withstand various sterilization procedures. Since they are biocompatible, they are chemically and biologically inert which is why they don’t interact with the human body fluids and tissues. Medical plastics are highly robust as they are extremely resistant to heat, radiation, and chemicals and they can thus, undergo various types of sterilizations
Medical plastics demonstrate high machinability and they are compatible with a variety of manufacturing processes. Some medical plastics demonstrate an increased level of tensile strength. E.g.: The highest tensile strength of Nylon is 12400 psi / 85.49 MPa.
Consideration Factors for Choosing Medical Plastics
Medical Device Environment
The first factor that should be considered when choosing medical plastics for your application is the environment of the medical device. A medical device is exposed to varying environments of temperature, chemicals, body fluids and tissues, radiation, etc. It is thus necessary to outline the medical device environment aptly so that correct medical plastics can be chosen.
The Extent of Contact
The next factor to be considered is the extent of contact of the medical device with the patient or the user. It is crucial to consider whether the medical device will be implanted or will be in direct contact when choosing medical plastics for the application.
Dimensional Stability
The functionality of the medical device is impacted by the medical plastics that are employed to manufacture the medical device. Medical devices require tight tolerances and thus, medical plastics need to demonstrate a high dimensional stability.
Application of Medical Polymers in Healthcare
Single-Use Items
One of the most common uses of medical plastics is as single-use items in the medical and healthcare industry. Medical plastics are inexpensive and it’s safer to discard than sanitize certain equipment. E.g. syringes, catheters, gloves, etc.
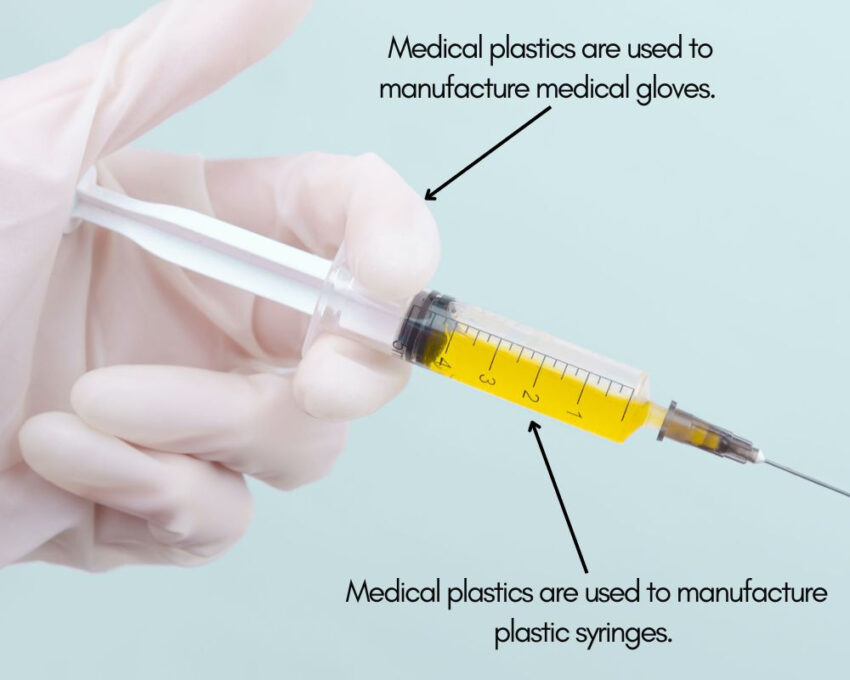
Medical Bags
Medical polymers are commonly employed to manufacture intravenous blood bags. Medical plastics are safe for storing blood and other fluids in a stable state. In addition, it’s inexpensive and can be discarded after use.
Sutures and Bandages
Medical devices such as sutures, bandages, or staples are often employed to close wounds. Medical plastics are an excellent material of choice due to their tensile strength and resistance to microbial growth.
Medical Tubings
Medical tubings are used in the healthcare industry for fluid management and drainage in the case of respiratory equipment, catheters, and more. Medical plastics are durable, flexible, and inexpensive which makes them an ideal material of choice for manufacturing medical tubings.
Implants
Medical plastics are commonly employed for manufacturing various types of implants such as heart valves, and knee and hip replacements. Plastic implants are comfortable, lightweight, and cost-effective. One of the most popular plastics that is employed for manufacturing medical implants is the durable ultra-high-molecular-weight polyethylene.
Prosthetics
Medical plastics are light-weight and comfortable which is why they are very popularly employed to manufacture medical devices in prosthetics. E.g. prosthetic limbs, artificial legs, knee implants, artificial fingers, etc.
Orthodontics
In orthodontics, medical plastics are often employed to manufacture braces or retainers. Since they are extremely flexible and easy to work with, medical plastics are used to create medical devices that are unique for each patient.
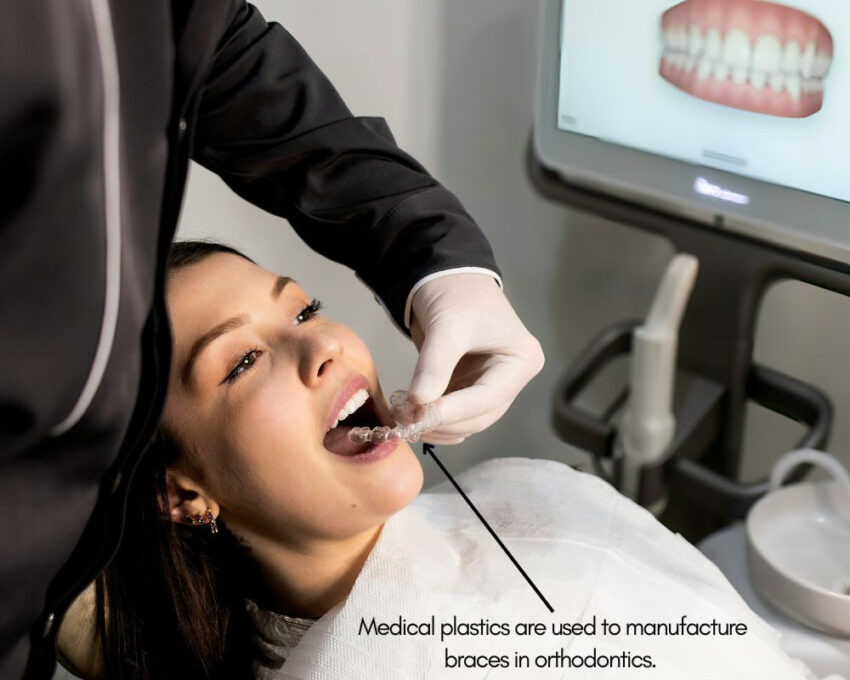
Regulatory Standards for Medical Plastics
Medical plastics undergo various screening procedures so that they can meet regulatory requirements. Several regulations control the use of medical plastics in the application of the medical and healthcare industry to ensure patient safety.
Medical device manufacturers must comply with the regulatory standards and requirements. When it comes to medical plastics, the key regulatory bodies are:
FDA Device Classifications
FDA categorizes medical devices into Class I, II, or III. These classes are based on the risk that the medical device poses.
- Class I medical devices are recognized as ‘low to moderate risk’ and generally do not require a premarket notification.
- Class II medical devices are recognized as ‘moderate to high risk’ and require a premarket notification requirement i.e. 510(k) submission.
- Class III medical devices are recognized as the ‘highest risk’ of all the classes. They require general and special controls as well as pre-market approval (PMA).
You can read more about medical device classification here.
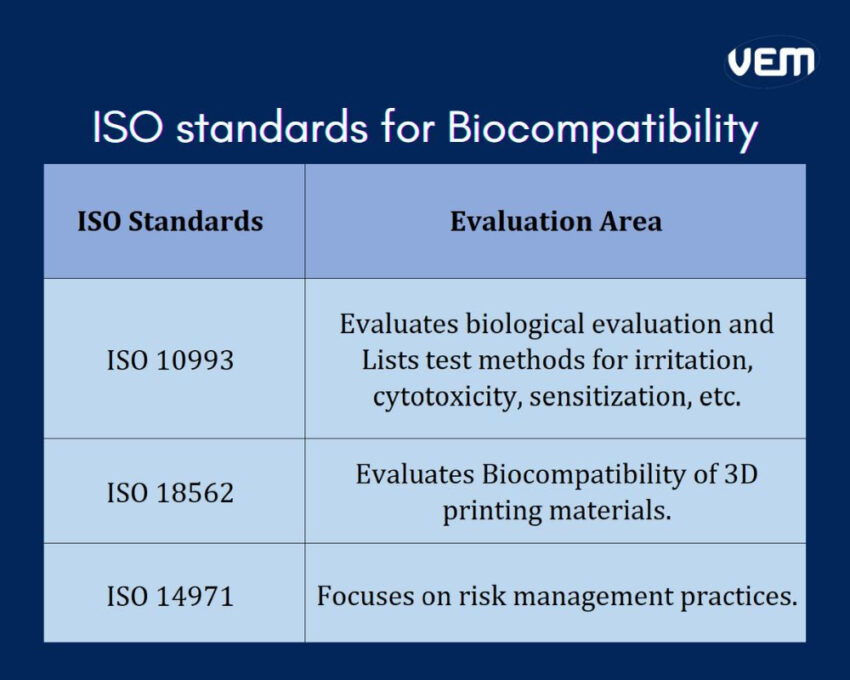
ISO standards for Biocompatibility
ISO, abbreviated for International Organization for Standardization standards provides guidelines to evaluate biocompatibility of medical devices. Medical plastics must be compliant with the standards of ISO. The following infographic illustrates the key set of standards for the evaluation of medical plastics:
FDA USP (United States Pharmacopeia) Class VI Passing
USP Class VI is a biocompatibility standard that is mandated by the United States Pharmacopeia. USP Class VI grade is required particularly for medical plastics that come in patient contact which exceeds 24 hours. For instance: implants, long-term catheters, etc.
You should note that USP Class VI testing is extensive and is considered to be the highest grade of biocompatibility. It indicates that the medical plastics used in medical devices are suitable for long-term exposure.
Key Aspects of Medical Plastics
Let’s understand some key aspects and essential properties of medical plastics in this section.
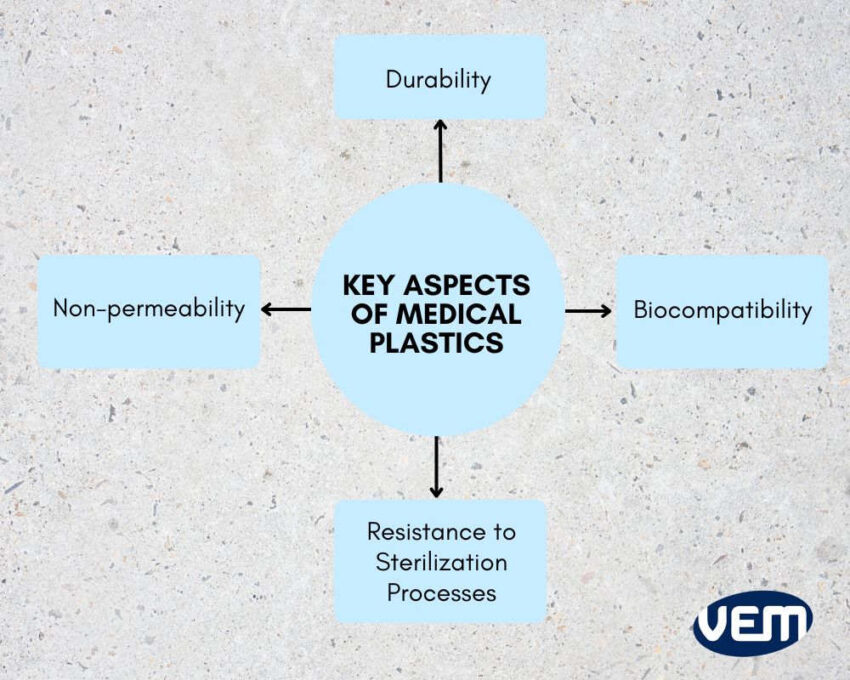
Biocompatibility
Biocompatibility ensures that the medical plastics are biologically and chemically inert within the body. If a medical plastic is considered to be biocompatible, it refers to the ability of the plastic to carry out the intended medical purpose with an appropriate host response. It’s thus crucial and critical that medical plastics are not immunogenic, detrimental, or toxic upon exposure to the body. The biocompatibility of medical plastics can be confirmed by ISO 10993 certification.
It requires that the medical polymers are compatible with the body tissues and fluids without causing any type of adverse effects thus, ensuring a safe body response. Let’s understand some factors that medical plastics are tested for:
Irritation and Sensitization
Medical plastics are tested through skin irritation studies to ensure that it does not cause irritation or swellings. They are also tested for sensitization to avoid any allergic reactions when implanted and evaluated for inflammatory responses in the body.
Cytotoxicity
Cytotoxicity detects the material’s ability to be toxic to living cells. Medical plastics are tested for cytotoxicity to understand their ability and the degree to which it can impart toxicity to cells.
Carcinogenicity
Medical plastics should not induce cancerous tumors upon implantation. They are thus tested for carcinogenicity.
Genotoxicity
Medical polymers are tested to identify genotoxins. This is carried out to ensure that the medical plastics that are applied to the manufacturing of devices do not cause damage to the DNA or mutations in the DNA.
Hemocompatibility
Medical polymers need to be tested for hemocompatibility if the device will be in contact with blood. This is to ensure that the medical plastics do not induce thrombosis or embolism.
Resistance to Sterilization Processes
Medical devices undergo repetitive sterilization which is why medical plastics that are applied to manufacture medical plastic parts must be able to withstand various sterilization techniques.
Medical plastics should be able to withstand heat and steam so that they can withstand autoclave and dry heat sterilization cycles, radiation so that medical polymers do not degrade due to gamma and UV radiations, and chemicals to avoid deformation and degradation.
When the medical plastic parts undergo any type of sterilization procedure, medical polymers shouldn’t change in appearance or physical and mechanical performance.
Non-permeability
Non-permeability of medical plastics is defined as their ability to act as an effective barrier against various substances such as water, gas, microbes, chemicals, etc. This is an especially crucial aspect in applications that require the handling, containment, and delivery of fluids.
Non-permeability primarily prevents the diffusion of substances through medical plastics. Let’s understand some examples:
- Medical plastic parts such as fluid bags, tubings, and catheters must be non-permeable to water and other liquids. They shouldn’t be able to transmit or absorb from medical devices.
- Medical devices such as oxygen masks and anesthetic equipment should be gas-impermeable. If such medical plastic parts do not restrict gas diffusion, it can lead to changes in the concentration.
Durability
Medical plastic parts must exhibit durability as they need to perform aptly throughout their lifetime. Thus, the medical plastics that are employed to manufacture medical devices must exhibit various attributes such as exceptional dimensional stability and high tensile strength. In addition, it must demonstrate a high level of resistance to impact, abrasion, and fatigue. Medical plastics must also be able to withstand exposure to sunlight, humidity, and various other environmental conditions.
Types of Medical Plastics
Acrylonitrile Butadiene Styrene (ABS)
Acrylonitrile Butadiene Styrene is a type of thermoplastic polymer that is a rigid and durable substitute for metal parts in medical devices. It also has a clean aesthetic appearance which is why it’s an apt choice for medical environments.
ABS demonstrates excellent abrasion, chemical, and corrosion resistance properties. In addition, it demonstrates a high tensile strength and heat resistance. ABS is employed to manufacture an array of medical devices such as non-absorbable sutures, tracheal tubes, drug delivery devices, etc.
ABS can undergo various medical device molding processes to manufacture a wide array of medical plastic parts. It is compatible with various manufacturing processes such as medical plastic injection molding, blow molding, 3D printing, and extrusion. In addition, it can also withstand medical device sterilization techniques such as moderate heat, gamma radiation, and ethylene oxide sterilization.
Polymethyl Methacrylate (PMMA)
PMMA, abbreviated for Polymethyl methacrylate, is commonly referred to as acrylic and is popularly known as bone cement.
PMMA demonstrates certain properties that are similar to glass. It’s transparent and can transmit and reflect light beams. In addition, it’s UV-resistant, waterproof, and offers weatherability at low cost.
PMMA has an elevated melting point of 200 – 250°C / 392 – 482°F. Due to its high level of melting point and an increased level of chemical resistance, it is commonly employed to manufacture endoscopic components. It’s also commonly employed to manufacture orthopedic implants, transparent medical ware, anesthetic masks, incubators, and viewing windows.
Polycarbonates (PC)
Polycarbonates are thermoplastic polymers that include carbonate groups in their chemical structure. Polycarbonates are durable and biocompatible.
They demonstrate a good level of toughness, high impact, thermal and UV resistance. In addition, polycarbonates exhibit excellent electrical and optical properties. Due to its high heat resistance, polycarbonates can resist deformation at high temperatures. They are also extremely pliable and can be formed at room temperatures without cracking.
It’s popularly employed in the medical industry to manufacture medical components such as IV connectors. It’s also applied to manufacture plastic gloves and syringes.
Polypropylene (PP)
Polypropylene is an extremely popular medical plastic as it can withstand extreme steam sterilization. It has a high melting point of about 1710°C / 3110°F which is why it can withstand the heat that is produced during autoclave sterilization. It is thus employed to manufacture medical parts where resistance to high temperatures is a priority. For instance: clear bags, disposable syringes, connectors, and oxygenator membranes.
It also offers excellent chemical resistance as it is stable to various acids, bases, and other solvents. In addition, Polypropylene is biocompatible, recyclable, and cost-effective.
Polystyrene (PS)
Polystyrene is one of those medical polymers that can be easily sterilized and is thus, extremely popular for manufacturing an array of medical devices! It also demonstrates excellent optical characteristics which is why it is especially applied to manufacture medical plastic parts in diagnostics such as petri dishes, tissue culture trays, test tubes, test kit cases, etc. In addition to being optically clear, it’s also resistant to chemicals and is an inexpensive material.
HIPS, abbreviated for High-impact polystyrene, demonstrates greater toughness which is why it is used to manufacture surgical instrument trays and emesis basins.
Polyethylene (PE)
Polyethylene is a versatile thermoplastic that can be applied to an array of medical applications. It’s durable and demonstrates a high impact and chemical resistance. In addition, it has a low moisture absorption rate and is biocompatible and biologically inert.
PE is also cost-effective and can maintain its structural integrity when subjected to various sterilization techniques.
Polyethylene is often applied for manufacturing medical and surgical implants. In addition, it is often employed for manufacturing fluid handling systems and lab equipment.
Polyethylene Terephthalate Glycol (PETG)
Polyethylene Terephthalate Glycol is a transparent and durable plastic that’s widely applied for medical applications. It’s machinable and can withstand various sterilization procedures. It can undergo sterilization procedures repeatedly without fading.
PETG is a safe plastic of choice for food containers. It is thus, popularly employed in the food preparation area of hospitals. It is also used to manufacture PETG sterilization trays.
Polyamide
Polyamide, commonly known as Nylon, is a synthetic polymer. Polyamides demonstrate high tensile strength and flexibility. They are thus, popularly employed to manufacture non-absorbable sutures.
They are considered to be strong medical plastics as polyamides are resistant to chemicals and abrasion. It also demonstrates anti-corrosive properties, high-temperature resilience, and stability.
Polyamides can be used to create medical plastic parts through various manufacturing processes such as injection molding, CNC machining, injection molding, or 3D printing.
Contact Us
It is critical to understand medical plastics aptly to achieve high-quality plastic machining for medical devices.
VEM Tooling and Medical has over 20 years of experience in mold design, injection molding, and medical device molding. We can help you develop your idea into a complete design. If you have a question about medical plastics or about manufacturing medical devices, you can contact us and talk to our experts.



