Medical device contact manufacturing is popular among most OEMs that want to benefit from the experiences of customer manufacturers. The entire industry has reshaped medical technology by overcoming various challenges to support innovations and its ever-increasing demands.
New innovations in technology and break-through have increased the use of medical devices. Today, the global market for medical device manufacturers is rapidly increasing and so is the requirement of companies and people to support the same.
The medical device industry has also become very competitive and it’s becoming more and more crucial for businesses to introduce high-quality and affordable products into the market before the competition does! This means finding the right partners for manufacturing solutions and support systems after the product enters the market
Contract manufacturing is a major segment of the rapidly evolving medical device manufacturing industry and it is estimated that the role of contract manufacturing will continue to augment. Contract manufacturing is a type of outsourcing service in which a third-party manufacturer creates either parts for a product or the complete product itself for an OEM (Original Equipment Manufacturer). In this article, we discuss medical device contract manufacturing services in detail.
What is Medical Device Contract Manufacturing?
The term ‘medical device’ includes an array of products that include equipment and devices for the operating room, nursing homes, intensive care units, testing laboratories, pharmacies, etc. You should note that medical devices are heavily regulated across the globe. In the United States, medical device compliance is regulated by the FDA (Food and Drug Administration). You can read more about it here.
Medical device contract manufacturing is a type of service in which a manufacturing company has everything in place including the compliances to build and manufacture medical devices or their parts that can be sold later by another company.
Typically, medical device contract manufacturers specialize in a particular process and their services can include product concept and development, process validation and verification, production, specialized manufacturing, and packaging. They can also manage the supply chain for the end customer.
Why should you choose medical device contract manufacturing services?
There are various benefits to opting for an OEM for contract manufacturing services. Let’s understand these advantages further:
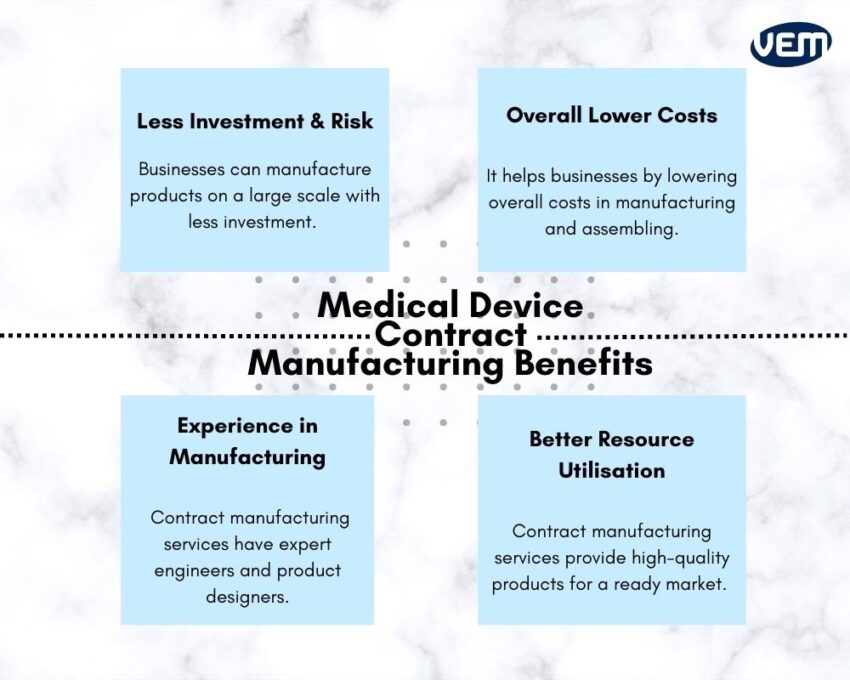
Less Investment & Less Risk
Contract manufacturing services enable businesses to manufacture products on a large scale with less investment. Companies are able to produce high-quality goods without requiring to invest in the set-ups of production facilities and techniques. Since the investment reduces significantly, it also reduces the risk.
Overall Lower Costs
Contract manufacturing helps businesses by lowering overall costs in manufacturing and assembling. This is especially helpful for international businesses that have production units situated in countries that have lower material and labor costs. In addition, contract manufacturers are also able to attain raw materials at competitive prices which helps to further lower the overall cost of the product.
Experience in Manufacturing
One of the major advantages of employing contract manufacturing services is their experience! They typically have expert engineers and product designers that can manufacture high-quality products quickly. They also have experts that can help to choose the right raw material and technique to manufacture your product.
Better Resource Utilisation
Contract manufacturing services provide high-quality products for a ready market. Companies can now focus on other aspects of the business such as R&D and attaining market share.
Why do some contract manufacturing projects fail?
Contract manufacturing has various advantages but there are some instances that lead to the failure of the same projects. Let’s discuss these instances:
Incorrect or Inadequate Information
Contract manufacturing projects are also referred to as build-to-print projects and one of the major reasons for such types of projects failing is not having the right information in place. It’s crucial to give detailed specifications and documentation to ensure accurate replication.
You should note that there is also a risk of intellectual property leaking out which is why it is essential to have a thorough NDA with your contract manufacturer.
Incomplete Documentation
If you are outsourcing your manufacturing project, then your documentation needs to be complete in terms of not just the design and specifications but also the bill of materials, and assembly instructions.
Low Quality Standards
If your contract manufacturer is not experienced, then there may be issues with respect to product design and quality standards. They may fail to follow the set standards which can lead to serious product quality issues.
How to select a Medical Device Contract Manufacturer?
Medical device manufacturing is highly regulated and a complex process. It is crucial that your project is handled by an experienced manufacturer to ensure seamless and efficient manufacturing.
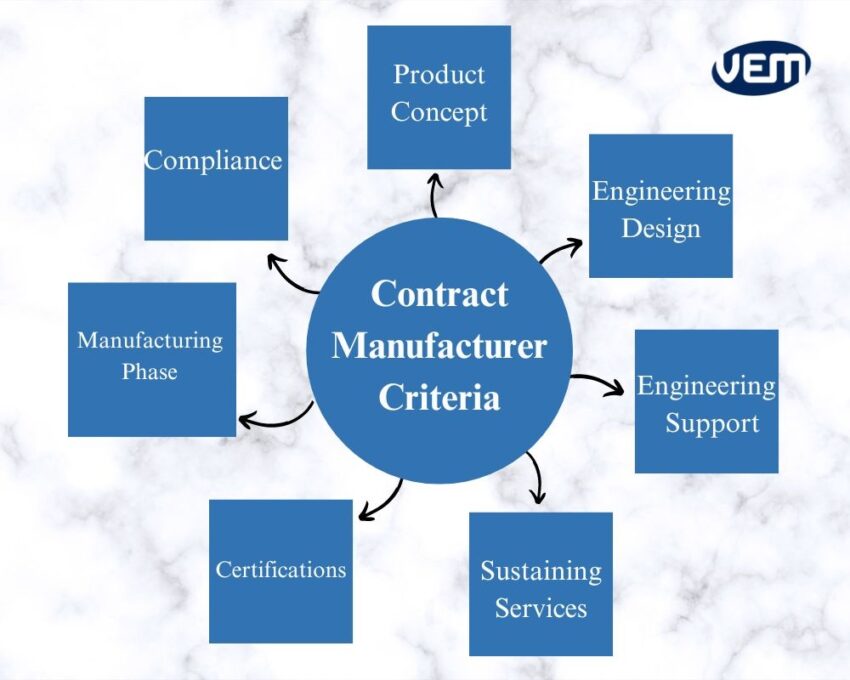
We have listed some factors that can help you select the best fit for your company:
Product Concept: Your original equipment manufacturer (OEM) should be able to take your ideas and conceptualize the products. You should be able to visualize the look and feel through 3-D simulations and conceptual renderings of the end product.
Engineering Design: The design engineers of your OEM should be able to transform the product concept and specifications into working prototypes to establish proof of concept. They should have an internal tool shop or a good partner to make tooling.
Engineering Support: Your OEM should be able to provide engineering support so that internal research and development efforts can be augmented. This also helps to launch the product faster into the market.
Compliance & Certifications: The design team of your OEM should be able to submit your prototypes to the appropriate regulatory agencies for certification and approval. It is imperative that they are experienced with documentation and have all the right certifications for approval.
Facility Certifications: You should also ensure that the manufacturing facility itself has ISO certifications and follows the current good manufacturing practices cGMP.
Manufacturing Phase: The manufacturing phase is yet another extensive phase and it is crucial to ensure that quality is a priority in each and every step. Your OEM should be able to manage the end-to-end manufacturing of your product. They should be able to procure the raw materials, manage the suppliers, set up the assembly line, and train the technicians and assemblers as required. In addition, the facility should be equipped with clean rooms for manufacturing, packaging, and assembly needs. You can read more about cleanroom here.
Sustaining Services: Your OEM should be able to provide the necessary services and support for product repairs, calibrations, and replacement parts when your product enters the market.
Engineering Expertise
Introducing a new, high-quality product into the market means that your contract manufacturer needs to have expertise in various engineering disciplines. We have listed below some of the engineering disciplines that may be required in your design and development process:
Industrial Design – This area of expertise identifies market opportunities and develops an appropriate solution to validate the same. It defines and determines the product’s forms and features.
Mechanical Engineering – Mechanical engineering is one of the broadest disciplines and it includes studying physical machines that can design and manufacture products.
Electrical Engineering – Electrical engineering is also one of the broader disciplines of engineering and it involves the maintenance and design of electronic equipment and electrical devices.
Embedded Systems Design – An embedded systems design expertise helps the manufacturer to control various machines using software engineering.
Printed Circuit Board Design – A printed circuit board design engineer oversees printed circuit boards (PCB) that are used in various electronic devices.
Manufacturing Engineering – This discipline focuses on the design and operations of manufacturing high-quality and competitive products.
Complete Medical Contract Manufacturing Services
In addition to the aforementioned aspects, you should select a medical device contract manufacturer that offers various phases of production.
An experienced and leading service provider should be able to guide and provide the following solutions:
- Design and Engineering Support
- Raw Material Procurement
- Project Management
- Quality Management Systems (QMS)
- Testing and Calibration
- Assembly
The manufacturing facilities and staff of your contractor should comply with the current good manufacturing practices (cGMP) and all the regulations such as FDA approvals and ISO certifications. They should also ensure consistent quality controls throughout the supply chain, assembly, and documentation services to maintain the highest standards.
You should note that it’s also a turnkey approach! Having a one-stop solution contract manufacturer helps bring the entire product design and development process together, under one roof. This reduces the risk of using multiple vendors at different stages in the design and development process.
Contact us for Medical Device Contract Manufacturing
A medical device contract manufacturer must be experienced from the initial concept to the final delivery of medical device equipment and assemblies.
At VEM-medical, we have experts that offer technological expertise, engineering support, and comprehensive manufacturing solutions to provide you with the best for your medical device contract manufacturing requirements.



