

The medical device industry is a huge industry that encompasses various products ranging from hearing aids, and laboratory equipment to X-ray machines. The manufacturing of medical devices is strictly regulated either by FDA (Food and Drug Administration) in the US or EMA (European Medicines Agency) in Europe. These agencies ensure the safety and efficacy of medical devices.
Medical device manufacturers must comply with the standards and guidance of regulatory agencies when designing, manufacturing, and marketing the product. The manufacturing of medical devices encompasses various aspects of the production process, from designing to packaging and shipment, and during all these processes, manufacturers must adhere to ISO standards, FDA regulations, and Quality Management systems. In this article, we explain in detail the manufacturing of medical devices and medical injection molding.

Fast cycle times and cost-savings are important manufacturing aspects but when it comes to manufacturing medical devices, quality control is of the utmost importance. Manufacturing medical devices demand constant insight into processes, machines, equipment components, and biologically safe materials. In addition, packaging validation which proves that a product is sterile when it ships is the final step to be mandated by FDA regulations.
The medical device industry caters to various healthcare segments such as therapeutics, monitoring, and diagnostics. The manufacturing sectors of medical devices include an extensive range of both, reconstructive devices such as hip and knee replacements as well as implantable monitors for cardiac and diabetic care.
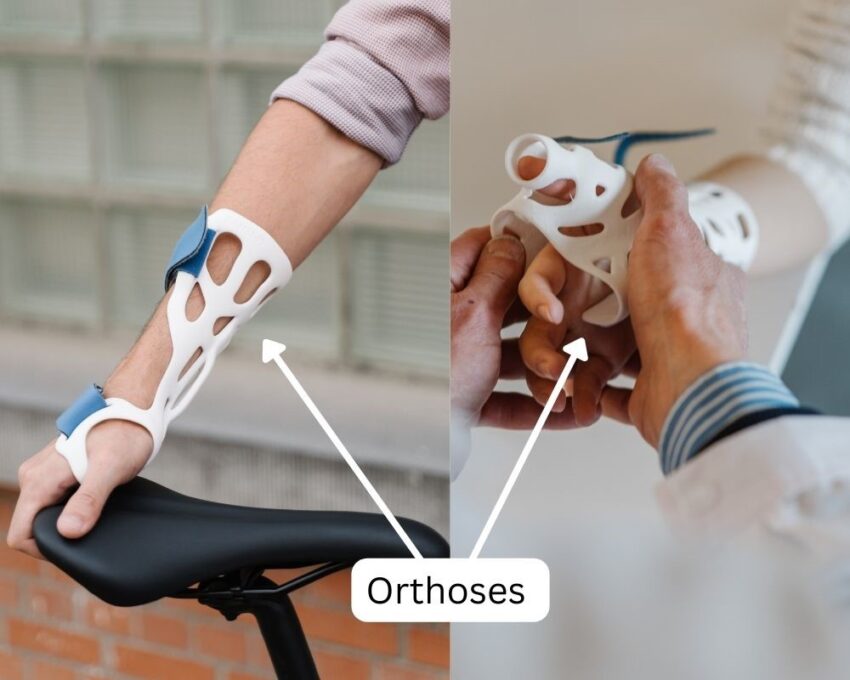
Orthopedics utilizes various manufacturing processes such as machining, casting, grinding, polishing, metal injection molding, and rapid manufacturing to develop reconstructive devices, orthoses, arthroscopy instruments, knee replacement, and hip and spinal implants.
The manufacturing of surgical instruments utilizes technologies such as micromachining to manufacture dilators, sutures, and surgical robotics.
The key technologies for diagnostic apparatus include imaging, IT, and micromanufacturing to manufacture endoscopic devices, ultrasound, and magnetic resonance instruments.
Manufacturing of cardiovascular devices includes technologies such as micro molding, and assembly to develop and create pacemakers, defibrillators, and drug stents.
Dental instrument manufacturing uses technologies such as machining, additive manufacturing, and 3D imaging to manufacturing equipment, implants, drills, and instruments.
Manufacturing of medical devices via injection molding or any other technique requires strict adherence to the processes and careful selection of materials to ensure that all standards of the regulations are met.
ISO regulations govern the outputs and processes of various industries, including the medical field. Let’s take a look at some of the regulations and compliances:
There may be other types of specific requirements regarding the product or application that may be needed, and some medical manufacturers are able to guide you by providing you with the correct and thorough documentation to meet these requirements.
Developing and gaining approval for a new medical device is often a slow and cautious process. The time frame completely depends upon the type of medical device being developed. The average time frame to develop a new medical device, all the way from concept to approval can take anywhere between 3 to 7 years and more. This time frame includes the entire device lifecycle such as research, development, and testing.
US FDA classifies medical devices based on the intended use of the device and also, upon the indications for use. You can understand these details further, in-depth on the US FDA website. Let’s understand the different classes of medical devices:
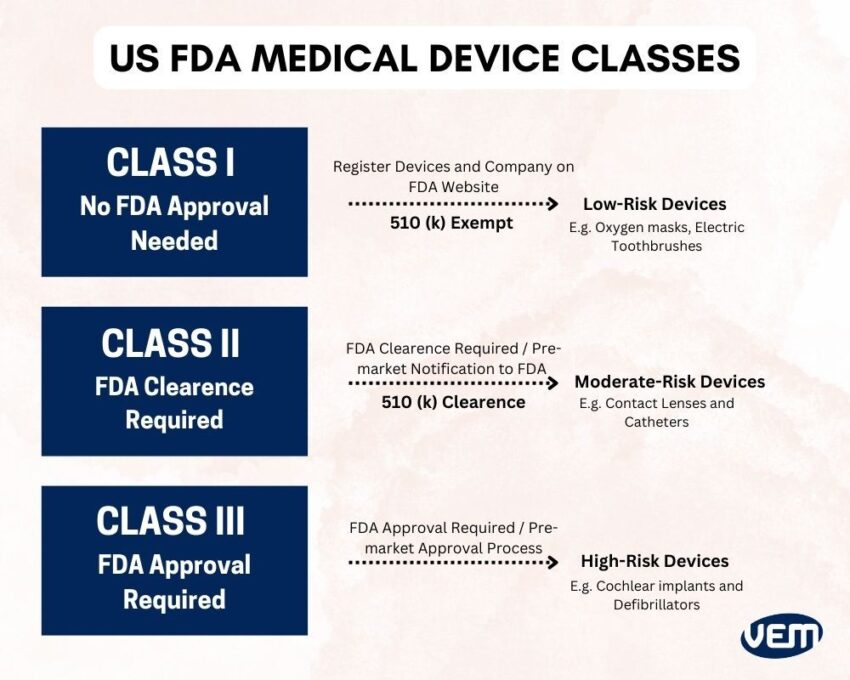
Class I medical devices are the type of devices that can pose only minimal harm to the user and are often simpler in design than Class II or III devices. Class 1 Medical devices are known as low-risk devices and include items such as tongue depressors, oxygen masks, non-electric wheelchairs, and electric toothbrushes.
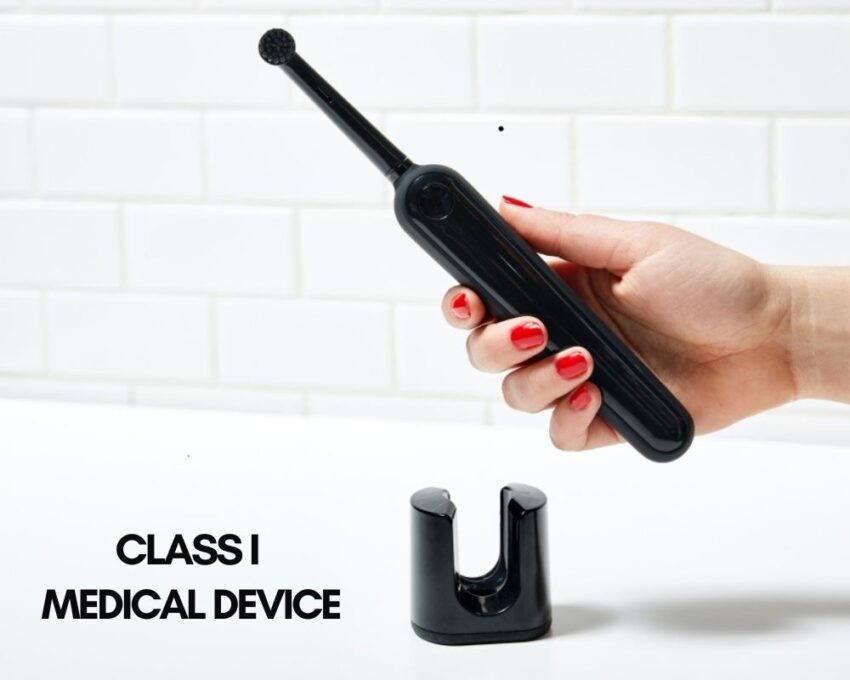
There is no FDA approval needed, however; the device and the company must be registered on the FDA website. The majority of class 1 devices that are non-invasive can be self-registered with the FDA. You should note that not all class 1 devices are exempt. If your device is classified as Class I but it’s not exempt, a 510k will be required for marketing.
Class II medical devices can pose more risk to patients than Class I medical devices. One of the major differences between Class I and II is the issue of premarket notification. Most class II devices require a 510(k) clearance. This clearance demonstrates that the particular device is substantially equivalent (SE) to one or more devices that were previously cleared by the FDA.
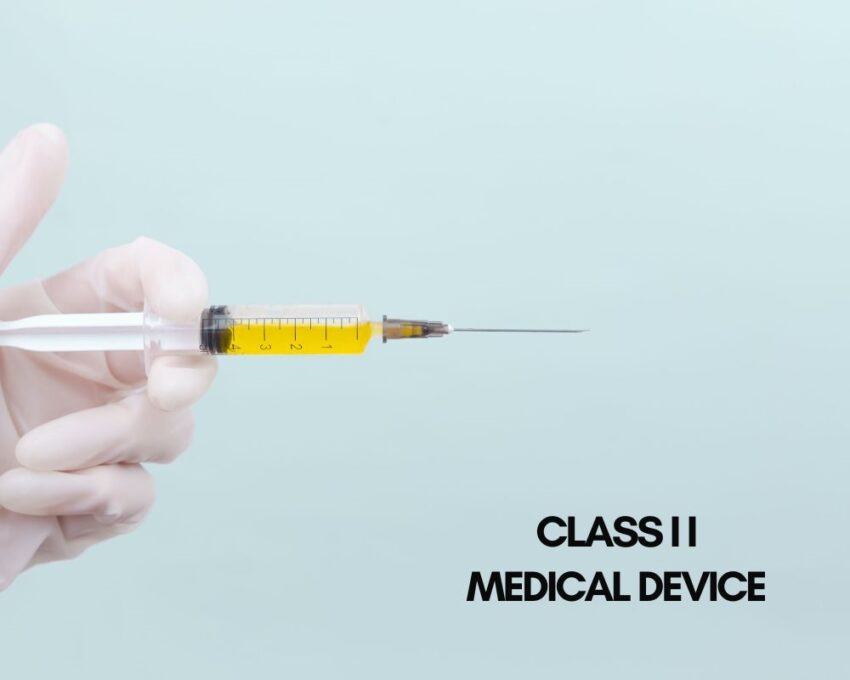
Class II devices are more in contact with the person or the user and thus, they pose a moderate risk. Examples include endosseous implants, contact lenses, syringes, OTC blood glucose monitoring systems, and catheters.
Class III medical devices pose the highest risk to patients than Class I and II medical devices as they are the most invasive medical devices. They require the most stringent checks during the development process in order to attain approval.
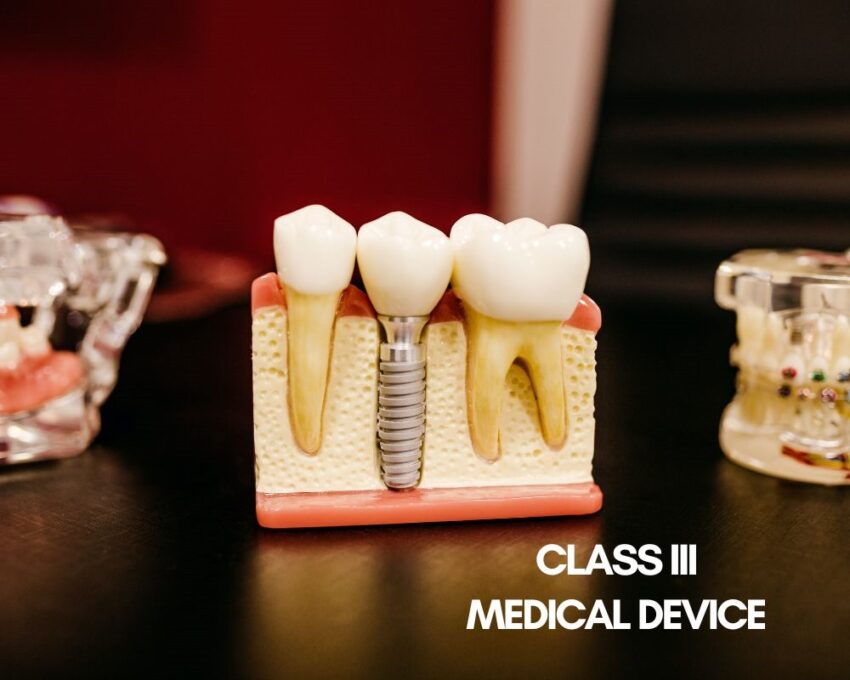
Class III medical devices include items such as cochlear implants, defibrillators, and dental implants.
The above FDA classification is recognized in the US whereas, Europe follows the European Union Medical Devices classification and Canada follows the Canada Health Medical Device classification. The classification is depicted below:


The main requirement for injection molding medical devices is biosafety and chemical stability since these items come in regular contact with the human body and medications. Thus, it’s absolutely essential that plastic resins are biologically inert so that they do not cause harm to the human body or leave toxic substances in organs and tissues.
Injection molding is popular because it’s inexpensive compared to other manufacturing methods but there are very limited ways in which plastic injection molding can be used to manufacture medical devices. The process of creating these products involves developing sterilization-friendly, accurate, and durable plastic parts, generally in huge quantities. Plastic injection molding is the most advantageous manufacturing process for medical devices in large quantities.
Injection molding is a popular manufacturing process for medical devices and other healthcare segments, as well due to its capability of being efficient and cost-effective. Some of the benefits that medical device injection molding provides are improved ergonomics and functionality, reduced weight, and lower cost.
The plastics used for manufacturing medical devices offer versatility and can be combined with metals to create medical products with enhanced attributes.
One of the top reasons to choose injection molding for manufacturing medical devices is that it is a very stable and reliable process. You should note that injection molding design and engineering can create and manufacture the highest quality medical devices.
Today, there is advanced modeling software that creates accurate tool designs. Additionally, with modern digital controls on plastic injection molding machines, the process parameters can be quickly tweaked for optimal performance. They can also be optimized to reduce the production lead time of injection molded medical devices to a minimum which in turn, helps to reduce the cost of your production.
Medical devices are often intricate and to make such a complex shape might require making an equally complex mold tool. Thus, injection molding processes require an initial product design and development investment. This needs to be considered before the manufacturing of any medical device however; once the tool is approved, it’s possible to produce a high volume of identical plastic parts quickly and economically. You should note that injection molding is thus perfect for more generic devices such as containers, tubes, or disposable single-use medical products like syringes.
One of the major advantages of choosing medical device injection molding is that there is a huge array of plastic resins to choose from for manufacturing. These resins have chemical, physical and mechanical properties that are known to manufacturers well enough thus, they can be chosen appropriately for their end use. You should note that today, there are resins that are heat stable, chemically resistant, strong, lightweight, biodegradable, and biocompatible.
Plastic resins are becoming increasingly popular for prosthetics, replacement joints, or other medical products that can be in permanent contact with the body. With such a wide variety of plastic available for injection molding, you can find the best fit for your medical device manufacturing.
One of the major limitations of medical device injection molding is its high initial tooling cost. However, tooling costs in injection molding should always be calculated as an investment. In order to ensure long-term efficiency, cost-effectiveness, and consistent production, it is important that the design of the tool is correct and that all aspects of tool design, construction, and overall process are carefully considered.
You should note that mold efficiency depends upon various factors such as cavitation, parting-line geometry, gating, venting, surface finish, and supporting automation. It’s important to ensure that the mold is robust enough to mitigate any type of minor variability that may arise in the injection molding process. Medical device injection molds also need to integrate seamlessly with equipment that pumps, mixes, injects, compresses, heats and ejects components.
Medical device injection molding also presents processing problems such as restrictions on part thickness (to avoid shrinkage problems) or de-flashing operations that can cause an additional cost. All these issues can be mediated if the tool and process equipment are designed expertly.
When injection molding is applied to manufacture medical devices, there are various considerations that should be taken into account! This applies not only to the design stage, where engineers examine material choices, but during all planning sessions, and the actual manufacturing process. A few of the most important considerations include:
Plastic resins that are used for manufacturing medical devices need to have certain physical properties. You should note that there are several grades of plastic resins that are available for medical device injection molding and medical device prototype development. Each of these resins has unique properties that determine its performance and its application.
Medical devices need to be capable of sterilization. These materials need to be resistant to contaminants and capable of being sterilized without undergoing degradation and be safe for use on humans. In addition, it should be heat and chemical-resistant.
Plastics vary in their strength levels, thus, it’s important to ensure that the material you select is apt for your application in terms of strength properties.
Since medical devices are exposed to bodily fluids and chemicals, they need to be durable and reliable even when exposed to liquid, vibration, heat, corrosive substances, movement, and other situations.
If plastic resins are fragile, they can become a hazard in the medical industry, thus, it is imperative to ensure that the selected materials should be shatter-proof.
Medical devices vary depending on the application thus, they have different FDA requirements. For instance: Implantable medical devices have different requirements than medical instruments and other devices that are used externally. You should review the FDA requirements for your application prior to selecting a material for your medical device.
You should ensure to choose a plastic material that can withstand your operating environment conditions. The environmental conditions determine requirements such as strength, temperature, chemical, corrosion resistance, and other factors.
Medical injection molding and insert molding are commonly used for medical device prototype development. However; not all plastic materials are compatible with both technologies thus, it’s important to understand the method type you will be using to manufacture before selecting the material.
Injection molding can be applied to manufacture various types of devices such as syringes and dialysis machine components. While it must be ensured that the medical device is manufactured as per FDA standards and is ISO 13485 compliant, you also need to ensure that the manufacturing company is the right one for your requirements. An experienced injection molding partner can help you ensure that your part cost stays low while maintaining the highest standards of quality. Let’s understand some of the points that you should keep in mind while screening to select your medical injection molding partner:
Cleanroom molding is an injection molding process of manufacturing plastic parts in a special room that is optimized to reduce the risk of contamination by dust, bacteria, and/or other particles. Cleanrooms have a constant positive airflow, use electric machines, and are devoid of any corrugated material that could cause dust. You can read more about how manufacturers maintain cleanrooms, in this article to ensure cleanliness.

It may, for example, be necessary to use a cleanroom for your medical device if the part is implantable, will come in contact with bodily fluid, or will be used in an operating room. If your device does not need to be cleanroom manufactured but does require a more controlled manufacturing environment, you should search for an injection molding partner that is flexible with their manufacturing environments. For instance: A Class 7 Cleanroom can be transformed using mobile enclosures over the plastic molding machine (by placing a curtained device over the area that offers positive airflow) and having machine operators wear a hat, gown, and mask.
An experienced medical device injection molding partner can guide you in determining the type of raw materials that can be used based on your product specifications. For instance: If your device will not be implanted or come into contact with a patient’s bloodstream, your plastic injection molding partner should steer you away from a Class 6, implantable-grade material and toward something more appropriate and affordable. On the other hand, if you’re creating a dental tool with a colored handle, the injection molding partner should ensure that you select the right FDA-approved, food-grade-contact material.
It is imperative that your injection molding company has the expertise and lays an emphasis on every detail of the project. Whether you need to create a smaller batch of specialized plastic parts or millions of plastic parts each day, you’ll want to partner with a medical molding company that can produce high-quality medical device parts.
Medical injection molding offers various benefits compared to other processes. If the correct plastic is chosen for the application, it can offer strength, and stability at a cost-effective price. Plastics can also be molded into the most complicated and complex molds that may not be possible with other materials. In order to understand, if plastic injection molding should be your choice, you should connect with an expert mold maker that can offer resin selection, turn-key assembly options, and program management.
At VEM Tooling, we are very effective at producing high-quality medical devices. Our team has vast experience in validating processes for medical device manufacturing via injection molding and is an expert in setting up a robust manufacturing process. If you are searching for a medical devices manufacturer and have queries, you can contact us and we will get back to you right away.